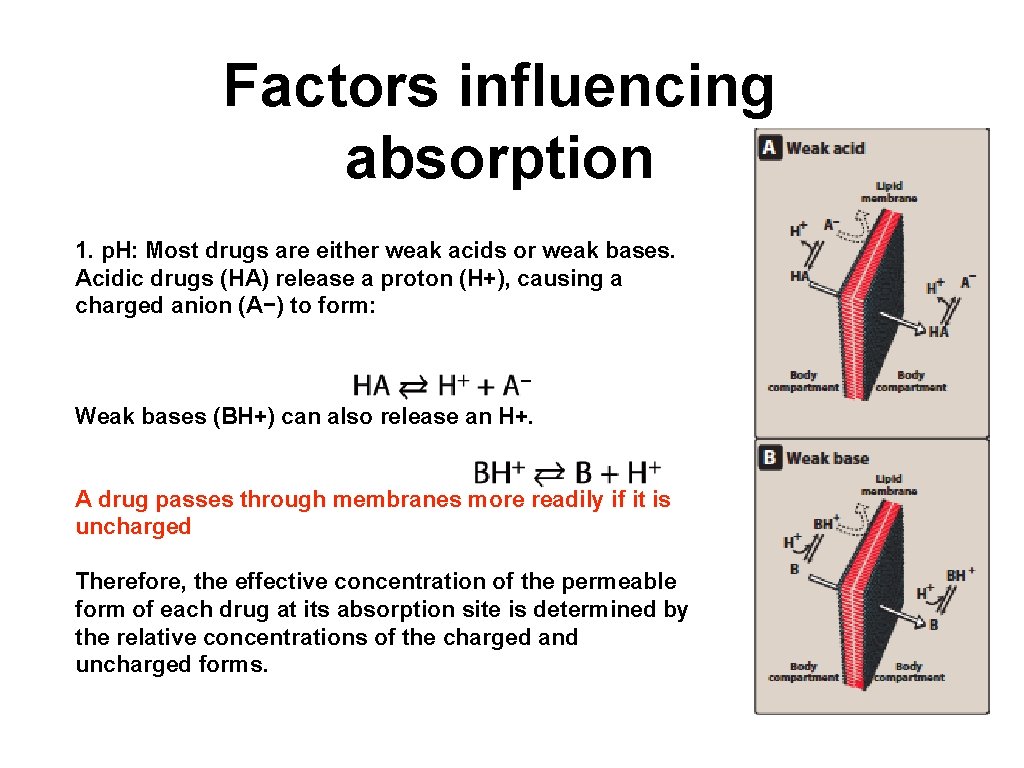
Most Drugs Are Weak Acids or Bases
These include paracetamol pKa 95 morphine pKa 99 and levothyroxine thy-. Drugs are often formed as a weak acid or base but this drug form is not always optimal for dissolution or absorption into your body.

Stem Engine General Chemistry Weak Acids And Bases Stemenginenotes Grade 11 12 A Weak Acid Is An Acid Which Partially Dissociates Into Its Ions In An Aqueous Solution In The Contrary A
In what chemical form charged or uncharged is cocaine snorted.

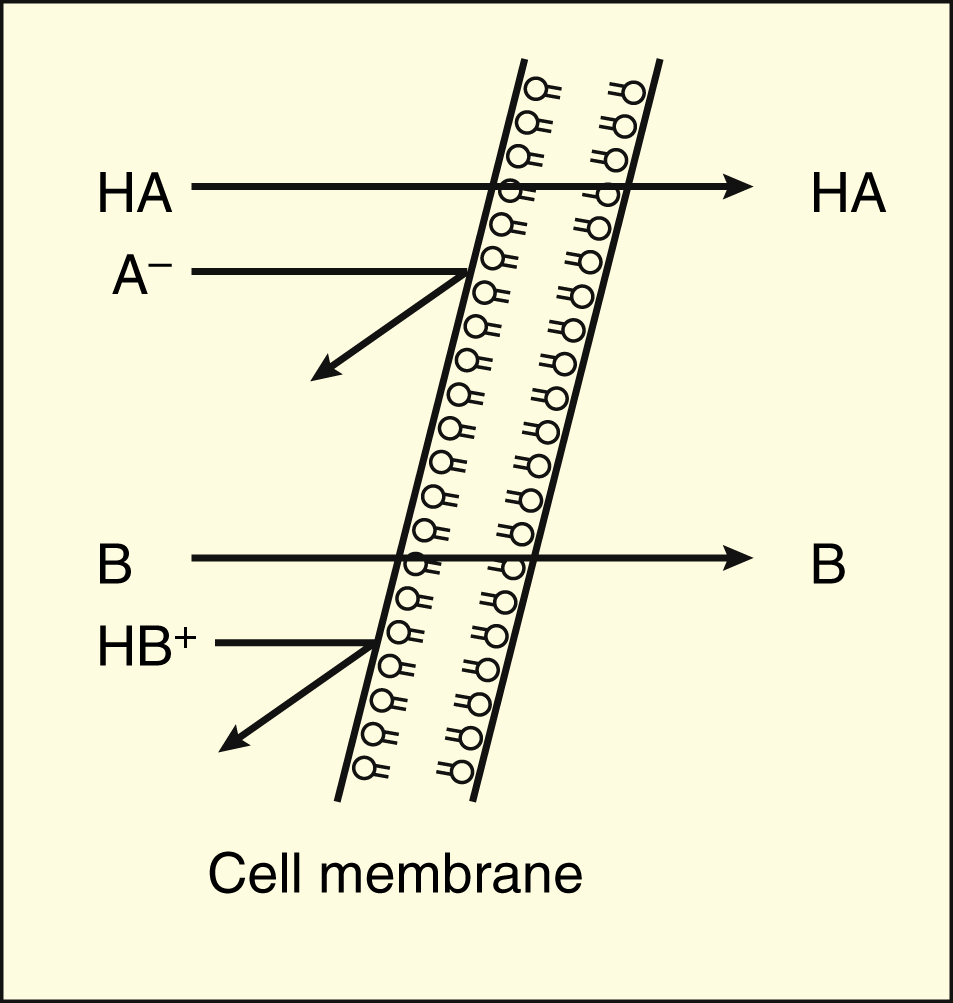
. Most drugs are weak organic acids or bases existing in un-ionized and ionized forms in an aqueous environment. Ionized molecules are usually unable to penetrate lipid cell membranes because they are hydrophilic and poorly lipid soluble. A number of common drugs contain the phenol functional group.
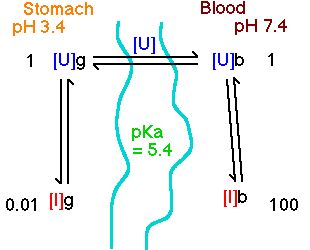
This is necessary because drugs need to be able to pass through cell membranes thats exclude strong acids and bases and dissolve in the stomach and blood thats favor. Most drugs are weak acids or bases that are present in solution as both the ionized and unionized forms. Terms in this set 49 bronsted lowry definition of acids and bases.
Many drugs are weak acids or weak bases. Unionized molecules are usually lipid soluble and can diffuse across cell membranes. Those that are weak acids ionise in water to give acidic solutions while those that are weak bases ionise to give basic solutions.
Unionized molecules are usually lipid soluble and can diffuse across cell membranes. Most local anaesthetics are weak bases with a pKa between 8 and 9 so that they are mainly but not completely ionised at physiological pH. Most drugs are weak acids or weak bases.
Also what is a basic drug. C 8 H 7 O 2 COOH C 8 H 7 O 2 COO- H Neutral aspirin. Most of the drugs are available as weak acids or weak bases.
A weak acid or base can exist in 2 forms charged ionized or uncharged unionized. They are absorbed in the undissociated lipid soluble form. Over 50 of all drug molecules used in medicine exist as salts most frequently as the hydrochloride sodium or sulfate salts.
1 Most drugs are either ____ acids or ____ bases. Similarly weak acid is absorbed at a faster rate from stomach pH 14 2. So the uncharged substances can be passed easily due to its lipid solubility.
Acids-substances charged or uncharged which is capable of donating a proton HA H2O. For drug absorption to be most efficient the properties of the drug itself and the pH of the environment where the drug is located must be considered. The dissociation of weak acids is suppressed by low acid pH.
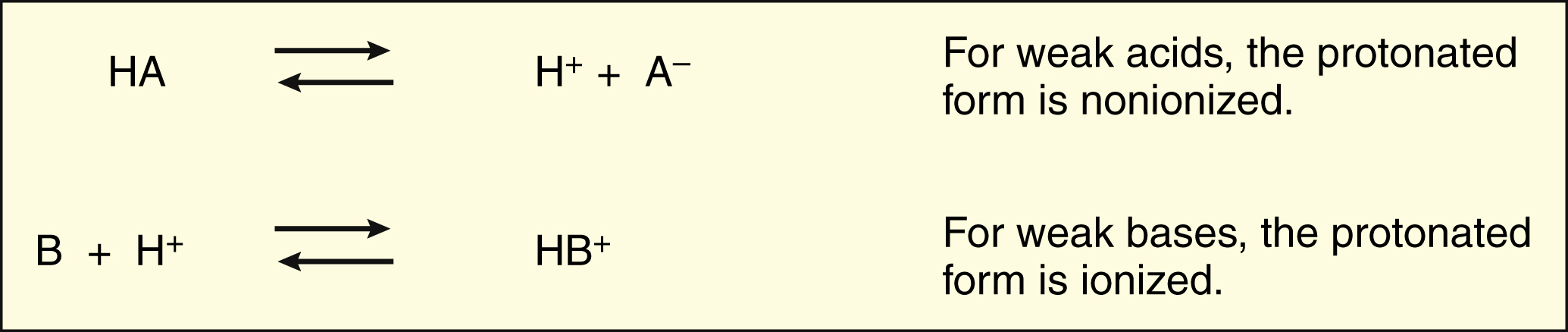
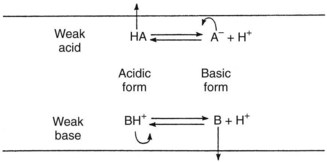
Most drugs are weak organic acids or bases existing in un-ionized and ionized forms in an aqueous environment. Most weak bases pKa bw 8-9 BH form predominates at physiological pH but too large to pass through the sodium channel B form penetrates the neuronal cell membrane accepts H from the cytoplasm binds to sodium channel as BH Removal of B. A weak acid is a neutral molecule that dissociates into an anion negatively charged and a proton a hydrogen ion Example.
Drugs that are weak acids will pick up a proton when placed in an acidic environment and will thus be un-ionized. The dissociation of weak acids is suppressed by low acid pH. The pre dom i nant form in.
In an aqueous environment acids and bases exist either in an unionized form or in an ionized form. Drug molecules that are weak acids Drug molecules that are weak bases where HA acid the drug molecule H 2 O base. Chemical characteristics of cocaine Cocaine is a molecule made up of C H O and N atoms.
Most drugs are salts of either weak acids or weak bases. It is a weak base the N has 3 bonds and in solution it exists in 2 forms in an equilibrium. H A- HA.
Thus theoretically one would expect a weak acid to be absorbed primarily in the stomach which has a low pH. The un-ionized form is usually lipid soluble lipophilic and diffuses readily across cell membranes. Drugs as Weak Acids and Weak Bases.
The un-charged species B penetrates the nerve sheath and axonal membrane and is then converted to the BH active form which then blocks the Na channels. Thus aspirin is a b ____ and pyrimethamine is a n ____. Carboxylic acids and may be precipitated from solution of the phenoxide by saturation with carbon dioxide.
Most drugs ionise in aqueous solution1 They are weak acids or weak bases. The majority of drugs are weak organic acids or bases. For example barbiturates are weak acids whereas amphetamines and opiates are weak bases.
Ionized molecules are usually unable to penetrate lipid cell membranes because they are hydrophilic and poorly lipid soluble. Weaker acids than carbonic acid H2CO3 which means that they do not react with sodium bicarbonate cf. They are absorbed in the undissociated lipid soluble form.
The weak base is absorbed at a faster rate from the intestine pH 750 8 this is because the basic substances cant be ionized in basic medium. Without absorption a drug cannot have a therapeutic effect so some forms require a. Most drugs are either weak acids or weak bases.
The free base and the acid salt see Figure 1. 21 Aspirin readily donates a proton in aqueous solutions and pyrimethamine readily accepts a proton in aqueous solution. In an acidic medium basic drugs are more charged.
The following reactions demonstrate how a weak acid or base is converted to its ionized form. What is the major factor that determines whether the weak acid or base is charged or un-charged. Theoretically weakly acidic drugs eg aspirin are more readily absorbed from an acid medium stomach than are weakly basic drugs eg quinidine.
Is cocaine a weak acid or weak base. Most drugs are ionizable organic compounds 75 weak bases 20 weak acids The remainder are neutral or quaternary ammonium compounds What is the Bronsted-Lowry acid-base method. Most drugs are salts of either weak acids or weak bases.
Most drugs are weak acids or bases that are present in solution as both the ionized and unionized forms.
Pharmacokinetics Dr Alia Shatanawi Pharmacokinetics May Be Simply

Drug Incompatibilites Ppt Download
What Are Some Examples Of The Most Commonly Used Weak Acids Quora

Drug Distribution Flashcards Quizlet
Pharmacokinetics Basicmedical Key
Pharmacokinetics Basicmedical Key

Section 1 Lecture 2 Pharmacokinetics Drug Ppt Download

Acids Bases And Drugs Ppt Download

Acid Base Chemistry In Medicinal Chemistry Youtube
2 Pharmacokinetics The Absorption Distribution And Fate Of Drugs Pocket Dentistry

Absorption Distribution Metabolism And Excretion Ppt Video Online Download
Ionization And Pka Value Medicinal Chemistry

Geriatric Pharmacology Ppt Download

Weak Acid Weak Base Reactions Acids And Bases Ap Chemistry Khan Academy Youtube

Drug Excretion Pharmacology Medbullets Step 1

Acids Bases And Drugs Ppt Download


Comments
Post a Comment